
INFORMATION FOR PHYSICIANS
50% Less Bleeding
Pre-clinical trials indicate that plasminogen-depleted plasma obtained by using the PlasFree ClearPlasma filtration device reduces the amount of bleeding by more than 50%.
Ongoing: A Pre-market, Multi-center, International, Double-blind, Randomized, Two-arms, Controlled, Prospective Clinical Investigation Assessing the Safety and Performance of ClearPlasma™ for the Treatment of Patients Undergoing Coronary Artery Bypass or Valve Replacement.
This study is ongoing in four different countries and more than 10 different sites.
For more information:
https://clinicaltrials.gov/ct2/show/NCT05542277?term=clearplasma&draw=2&rank=1
Completed: Pre-market, multi-center, international, double-blind, randomized, controlled, prospective, first-in-human clinical investigation ClearPlasma Device, in which Patients presenting with acute upper gastrointestinal hemorrhage (AUGIH).
PLAS-FREE has demonstrated ClearPlasma ability to reduce bleeding in multicentral, double-blind randomized control clinical study on patients suffering from upper gastrointestinal bleeding (UGIB) done in Israel and Europe. The Use of ClearPlasma reduced the mortality events to zero (0) compared to 2 deaths that were reported in the control group. In addition, ClearPlasma reduces rebleeding events and minimizes bleeding compilation compared to the control groups.
Laboratory analysis of blood tests demonstrated significant differences in the ability of the ClearPlasma to generate more stable clots that reduce blood loss and prevent additional re-bleeding events compared to the control group. Furthermore, transfusion duration and the procedure time were shorter in the ClearPlasma vs control group (55.39 vs 72.42 minutes). In addition, ClearPlasma reduced hospitalization duration by X>17%.
When analyzing the data on transfused units of blood products, there is a trend in the use of fewer units of concentrated red blood cells in favor of the ClearPlasma group (1.43 vs 1.62 units), and no need for transfusion of the platelet units in the ClearPlasma group (0% vs 7.69%).
As far as the safety analysis is concerned, it is worth mentioning that there were no events during transfusion in the ClearPlasma group, while 2 AEs were reported in the control group. All in all, ClearPlasma was proven for the first time as a new and effective therapeutic modality for massive bleeding patients.
For more information:
https://clinicaltrials.gov/ct2/show/NCT04174989?term=clearplasma&draw=2&rank=2
Completed: Open labels phase first-in-human clinical investigation ClearPlasma Device, in which Patients present with acute upper gastrointestinal hemorrhage (AUGIH).
The company has successfully completed open-label phase of a clinical trial evaluating the safety and efficiency of ClearPlsama on upper gastrointestinal bleeding patients in Israel. We found efficacy indications using ClearPlasma on those bleeding patients which were demonstrated by reducing the time of bleeding, decreasing the number of platelets units needed for halting the bleeding, and finally, in 2 out of 5 patients, ClearPlasma helped reduce the hospitalization time. No adverse events or thromboembolic events were found using our technology.
Please find attached Investigational IFU:

Pre-Clinical results
ClearPlasma™ efficacy
ClearPlasma™ was tested in several animal models with massive bleeding
1. Tail Bleeding assay is widely used as an in vivo assessment of haemostatic action in rodents (1). The company has performed several experiments utilizing this model and has demonstrated that ClearPlasma™ reduces the amount of bleeding by more than 50%.
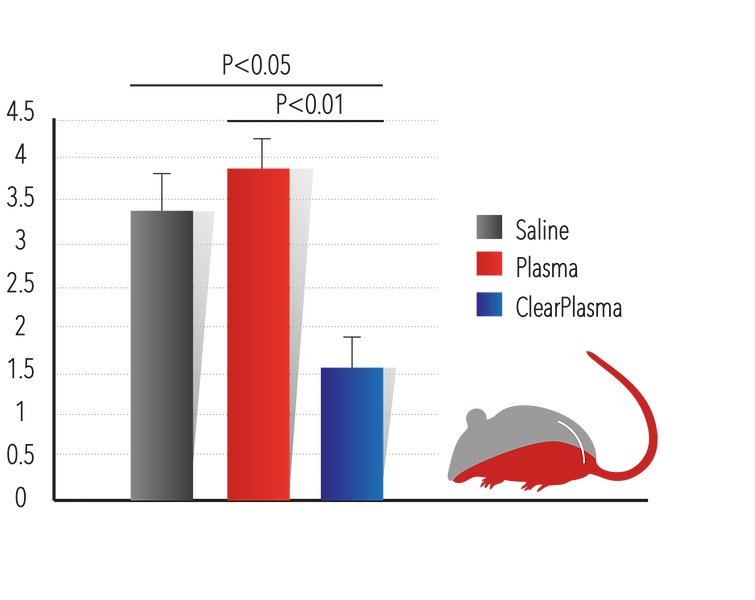
ClearPlasma™ was Reduces the amount of bleeding in more than 50%. The blood cell pellet from the bleeding test was centrifuged and the supernatant was aspirated. Pellet size was then measured using a ruler. A. Pellet size results for each mouse; B. Statistical analysis of pellet size measurement. Statistics were calculated using One-way ANOVA followed by posthoc LSD/SCHELF( p<0.05 considered significant). (N=20 for each group) .
2. Liver lacerations model- this model mimics trauma sustained to the liver. These trauma events can occur through either a blunt force such as a car accident, or a penetrating foreign object such as a knife. The company exmined ClearPlasma™ using this model in pigs. The Company demonstrated that plasma transfusion using ClearPlasma™ reduces the amount of bleeding by more than 50%, improves the hemodynamic parameters and benefits the animal condition.
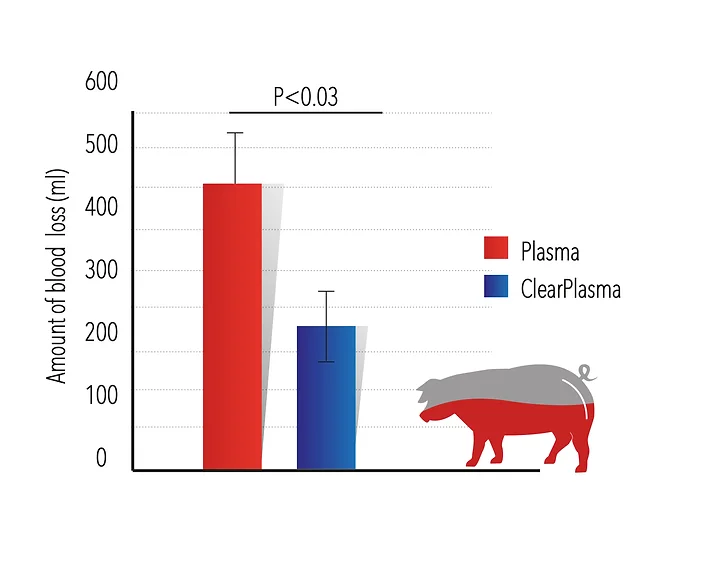
ClearPlasma™ safety
The company examined the safety of the device and the improved plasma in several aspects:
Its effect on thromboembolic events, Pyrogenicity or Haemolysis of red blood cells. The company demonstrated that ClearPlasma did not increase the levels of thromboembolic events, fever or Haemolysis.
